HENRY NOWICKI
Introduction
There has been quite a bit of media and political attention focused on groundwater and drinking water containing perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS). These compounds are synthetic, man-made chemicals that are highly persistent and slow to degrade in the environment. They are considered to be emerging contaminants by US EPA. These chemicals have historically been used industrially as emulsifiers in the manufacturing of fluoropolymers. Because of their unique ability to withstand water, grease and high temperatures. PFOS and PFOA have been used in very specific applications demanding these characteristics, including paper and cardboard food packaging, insecticides, electronics, stain repellants, paints, plumbing tape, firefighting foam, non-stick cookware coatings, and de-icing airplanes at 160 US military sites and commercial air flight locations.
Production of PFOA and PFOS was phased out in the early 2000s. Substitutes for phased-out PFOA and PFOS are also fluoroalkyls of unknown toxicity. By this time, large quantities of these chemicals had been released into the environments surrounding locations where they were manufactured and used. Investigations into these environments have detected PFOA and PFOS in drinking-water supplies in Hoosick Falls (NJ) Decatur (AL) Oscoda (MI) and Little Hocking (OH) as well as others near former manufacturing locations. PFOA and PFOS were added to US EPA’s Unregulated Contaminant Monitoring Rule 3 (UCMR3), which was promulgated in 2012. Monitoring activity conducted under UCMR3 has also revealed detectable levels of PFOA and PFOS in drinking-water supplies across the country.
Given the widespread usage of these chemicals (including usage in non-stick cookware coatings) it is not surprising that PFOA and PFOS have been detected at low levels in blood samples of the general US population. These contaminants are readily absorbed by the body; once ingested they tend to concentrate primarily in blood serum, liver and kidneys. Within the body, PFOA and PFOS have a half-life of 2.3 years and 5.8 years, respectively. Because of this relatively long half-life, repeated exposure at very low levels can result in accumulation in the body at levels that result in adverse health outcomes.
Epidemiological studies of workers exposed to high levels of PFOA and PFOS, and residential populations near manufacturing facilities, have demonstrated a positive association between serum concentrations and increased cholesterol, decreased bilirubin, low birth weight, immunological effects and cancer. In addition to epidemiological studies, animal studies of rats, mice and monkeys exposed to PFOA and PFOS have shown increased liver weight, liver hypertrophy, necrosis, developmental/neurodevelopmental delays, decreased spleen weight and delayed puberty.
Mechanism of Fluoroalkyl Adsorption
Coal-based and coconut-based activated carbons do not adsorb fluoroalkyls as well as they do chlorinated-, brominated-, or iodinated-organics. There are technical reasons for this phenomenon. Coal- and coconut-based activated carbons are the Best Available Treatment Technology today. Reverse osmosis (RO) is also used, but RO produces a concentrated waste stream and consumes a significant amount of water. A physical adsorbent like new lignin-AC would not have these problems. A solution for this fluoroalkyl problem is with a new activated carbon that can provide higher adsorption energy sites than coal- and coconut-based commercial activated carbons. Fluorine is the most electronegative element in the halogen family. So, fluorine’s outer shell electrons are not de-localized by adsorption forces provided by coal- and coconut- activated carbons. Whereas chloro-, bromo-, and iodo-organics outer shell electrons are delocalized by coal- and coconut-shell commercial activated carbons. Electron de-localization of non- fluoro halogen atoms in molecules results in partial positive and partial negative charges (electron deficient and electron rich spaces) which leads to inter-molecular attractive electrostatic forces and larger conjugated molecules that provide premature condensation in a gas phase and precipitation in a liquid phase application in activated carbon pores or coming out of water before the halogenated organic reaches saturated concentration. Fluorine does not allow de-localization of its electrons. They are not polarizable due to its low refractive index. Thus, you need higher AC pore concentrations before precipitation or removal from water is observed.
To solve this emerging fluoro- organics problem and other Ultra trace-trace real world problems that need to be solved, such as carbon dioxide and methane physical adsorption and desorption, our laboratory has made a new activated carbon from lignin feedstock. This lignin-activated carbon adsorbs fluoro-organics like 1,1,1,2- tetrafluoroethane (TFE) better than present coal-or coconut-based activated carbons.
Lignin is a waste by-product of cellulosic biorefinery industry. Lignin is a close second place most abundant natural polymer after cellulose. Lignin has no significant value; most of it is burned. There are presently 300 million tons of lignin available and its availability increases at a rate of approximately 20% per year. Biorefiners can use agricultural wastes: forest woods, corn field waste or stover, straw, sorghum, begasse, rice hulls, algae, etc. to isolate cellulose and lignin from the cell walls. Lignin and cellulose are 1:1 in stoichiometric concentrations.
We report, here, the transformation of lignin from hard wood trees to lignin- activated carbon and its comparative adsorption of 1,1,1,2-tetrafluoroethane (TFE). Small molecules are harder for activated carbon to adsorb than larger molecules. TFE is an excellent probe.
Each feedstock to manufacture commercial activated carbon has a pore or adsorption space distribution that reflex the feedstock. Presently, the major feedstocks are wood, coal family members, and coconut shells. Activated carbon can be made from any feedstock that contains fixed carbon. However, a requirement for commercial scale feedstock needs to be available, preferably at point sources, low cost compared to competitive present feedstocks, with few competitors for feedstock, a stable supply of feedstock, and feedstock suppliers’ benefits from working with activated carbon manufacturer and will make long-term contracts. Lignin meets all of these ideal criteria to be a major feedstock to manufacture activated carbon.
Lignin to – Activated Carbon Results
Lignin was transformed into lignin-activated carbon using two thermal processes – carbonization and steam activation.
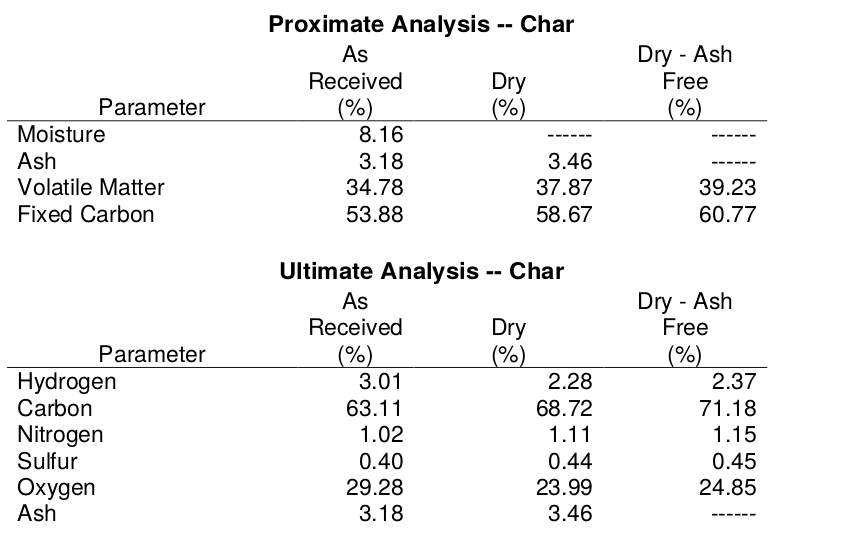
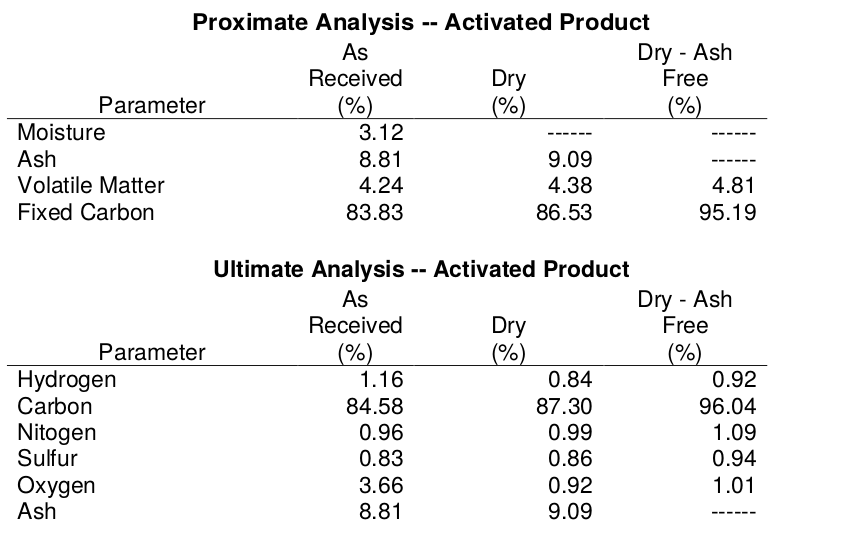
The tables below provide results for classical Proximate- and Ultimate- test packages for lignin-char and lignin-activated carbon. Typically, commercial activated carbons have negligible oxygen. Lignin is rich in oxygen and lignin-AC has significant oxygen content.
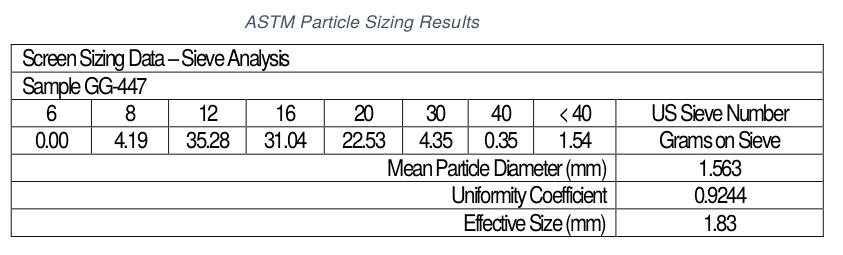
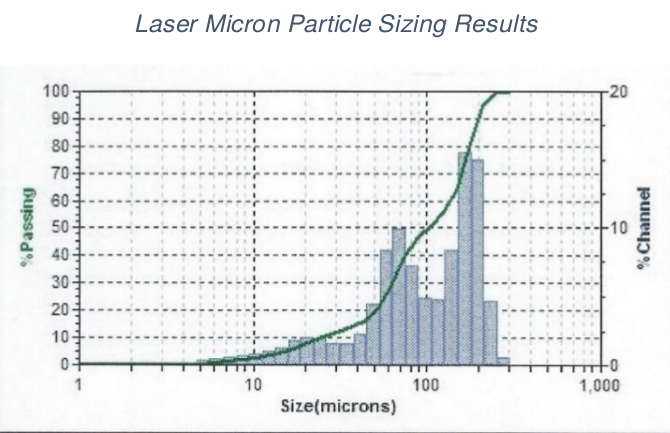
ASTM particle sizing with US mesh screens for starting lignin and laser micron particle sizing for product lignin-activated carbon are provided. These particle size differences are important.
Advanced GAED Performance Testing
Gravimetric Adsorption Energy Distribution or GAED full performance characterization provides full distributions of adsorption energy sites, from lowest to highest in calories per milliliter pore volumes. Smaller pores provide higher adsorption energy sites needed for fluoro-organic adsorptions and other Ultra-trace-trace loading applications.
Previously, extensive information was provided for GAED testing for advanced carbon performance evaluations (1). To summarize each GAED sample tested, can be powder, granular, pellet, or composited forms of activated carbon. GAED provides isotherms for any compound of interest for vapor phase- or aqueous phase-applications. Every GAED tested sample has its ASTM density and water content determined by heating each sample at 150°C for 3 hours. Apparent density enables providing adsorption data on a volume or mass basis. When a dry sample is put into GAED sample compartment it is heated to 250°C with flowing argon gas to protect it from oxidations for 25 minutes. Weight loss at 250°C is recorded and is in final GAED report. Losses below 8% are consider clean, well kept carbon.
Table 1 below provides GAED aqueous phase Carbon Characteristic Curves -Cumulative Basis performance for three lignin-activated carbon samples In Kiln for 25-, 28-, and 32-minutes, and coal- and coconut-based benchmark activated carbons. 25-minute char activation produced best product, GG-447A2.
Table 1 provides thirty data points for each sample; far left column is adsorption energy in cal/cc and five samples (columns) reveals the pore volume for each adsorption energy value for each sample.
Activated carbon adsorption sites needed to remove fluoro-organics are ≥ 25 cal/cc. As seen in Table 1, lignin-activated carbon out performed coal-and coconut- present activated carbons in columns 5 and 6, the last two columns to treat fluoroalkyl contaminated water.
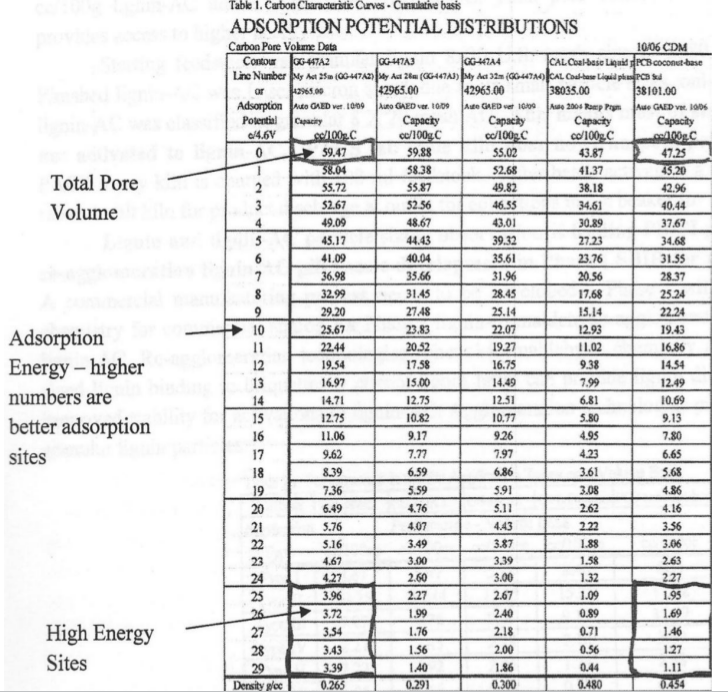
From 500 adsorption and 500 desorption data points the computer selects 30 data points to represent the Characteristic Curve shown in Figure 1. This data above is volume per mass adsorbed. Most activated carbon adsorbers are volume of adsorbate per volume of activated carbon like Figure 1, Volume based Characteristic Curve below.
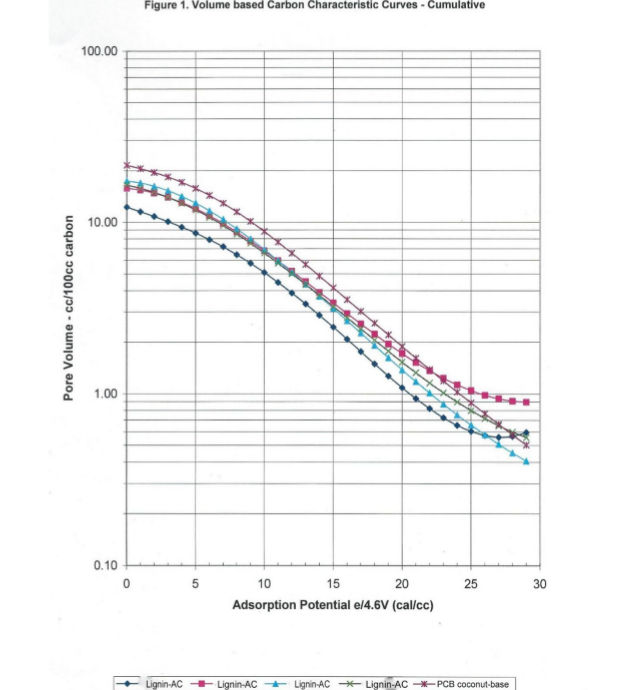
Activated carbons have some 2,500 commercial uses that can be placed in six activated carbon performance types. This is shown in Table 2a below.
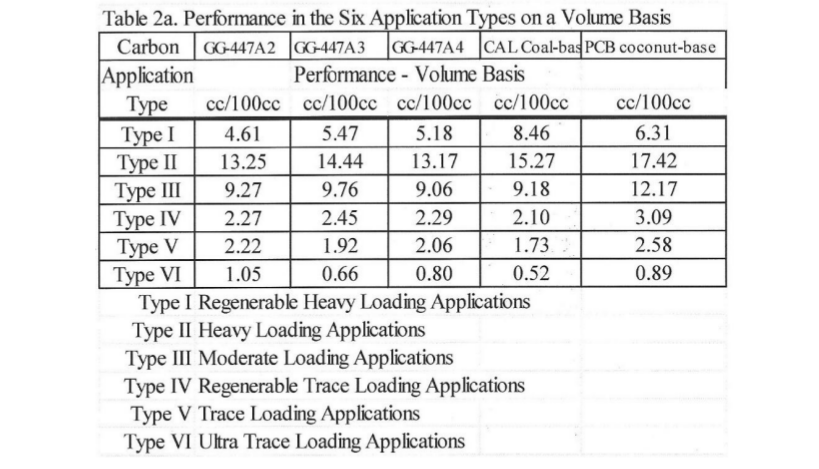
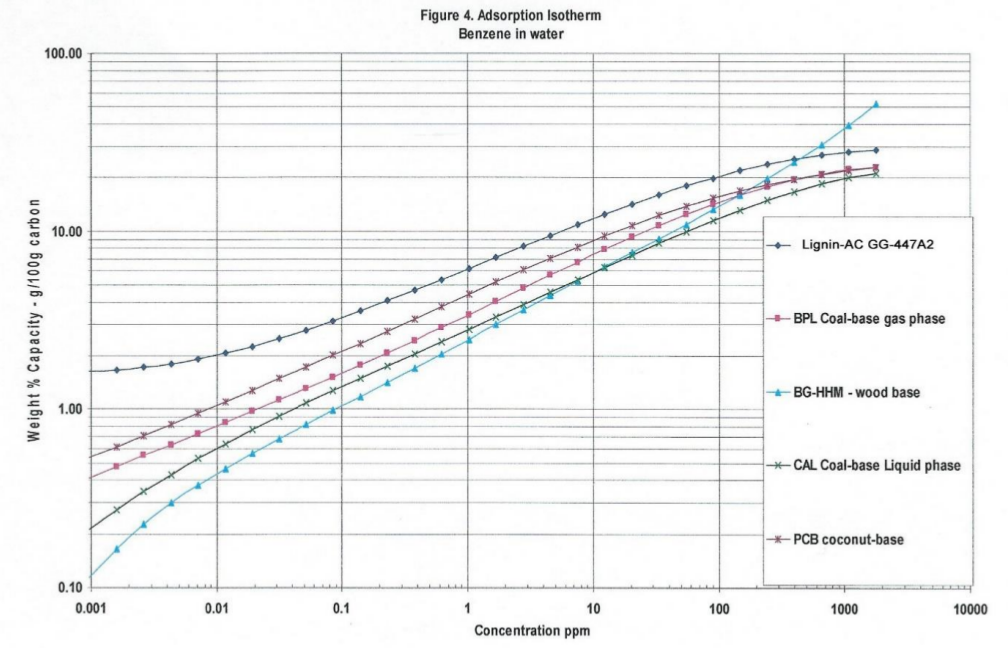
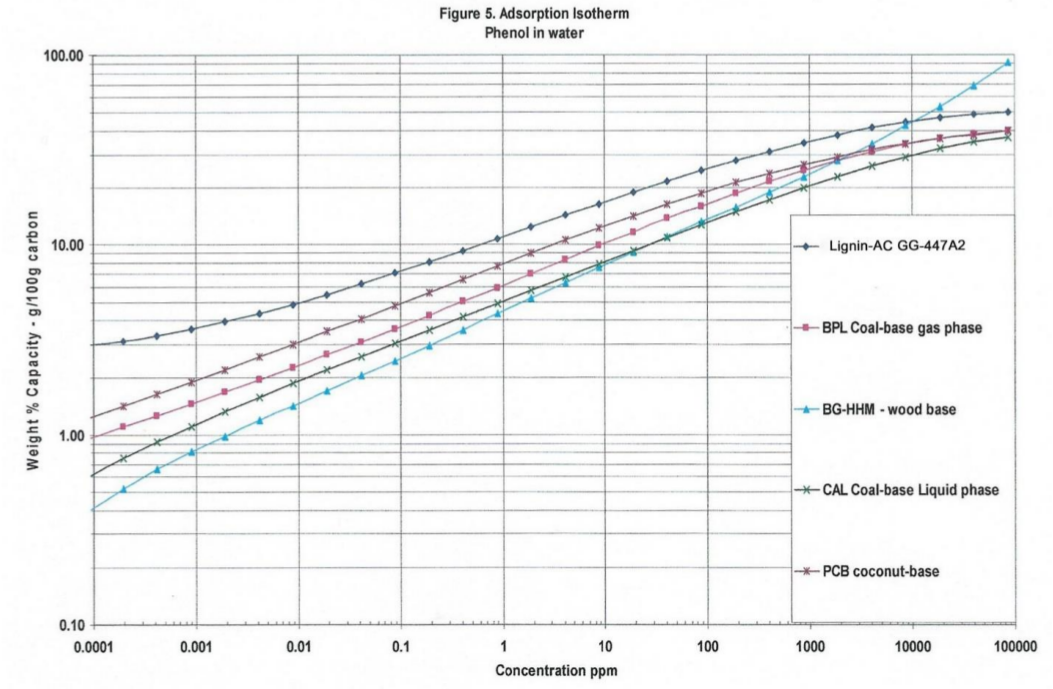
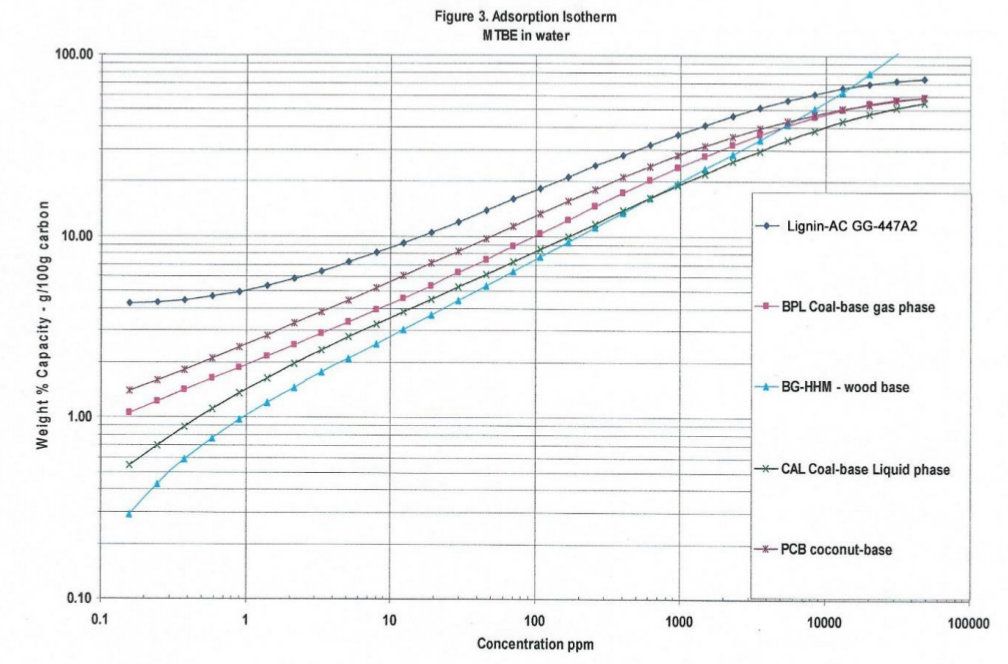
Note: Differences in density from last row of the data in Table1, lignin -AC is 0.265 gm/cc and coconut-AC is 0.454 gm/cc, so Table 2a 100 cc of lignin-AC is 26.5 grams and 100 cc coconut-AC is 45.4 grams. With less lignin-AC there is more performance of TFE adsorption isotherms. The standard GAED report provides three isotherms for aqueous phase applications. These isotherms are shown in Figures 3, 4, and 5. Methyl-tertiary-butyl ether, a difficult molecule to adsorb due to its water solubility, needs high adsorption energy (cal/cc) sites, benzene and phenol in water are adsorbed well by activated carbon.
In all three isotherms for target organics (methyl-tertiary-butyl-ether, benzene and phenol) lignin-AC outperformed coal-and coconut-AC in the 0.001- thru 1.0 ppm concentration range. Polanyi model is the basis of GAED. Polanyi model enables the determination of isotherms for any compound of interest for vapor- or aqueous- phase activated carbon applications.
Discussion of Results
Fluoroalkyl organics provide benefits and un-intended consequences. For instance, they provide non-sticking frying pans, water repulsion on fabrics, and are used to de-ice airplanes. However, they are being detected in ground waters. About half of US drinking water originates as ground water. There are claims of cancer clusters associated with these fluoroalkyl at user and manufacturing contaminated sites.
Lignin-AC outperformed coal and coconut-AC. But this work was designed to provide proof-of-concept and not optimization of process. What is now needed is a bona fide optimized commercial manufacturing process. Lignin is amenable to commercial processes.
Lignin-AC outperformed coal- and coconut-AC in all 6 types of AC applications. It is a candidate to solve Ultra-trace or Type 6 applications. There is obvious need for a Type 6 activated carbon.
The iodine number for lignin-AC was 1801.
The iodine number for the coconut-AC was 1200.
The difference in particle sizings of lignin and lignin-AC are very important. Starting lignin was 8 X 20 US mesh or 2,380-841 microns, and finished lignin-AC was 4.71-120 microns. This tells us granular lignin was converted to powder in the activation process. Present work is to keep granular lignin as granular activated carbon.
For other laboratory testing service, method development, R&D, software or consulting service needs, contact:
George@pacslabs.comTELEPHONE: (+1)
For Carbon Conferences or Short Courses in public sessions or at your time and place, contact us.
PACS provides testing, courses and consulting at the client's time and place, and twice a year carbon conferences.
PACS Positions Available:
- Laboratory chemist for activated carbon group. Will train!
PACS has positions available for professional scientific service providers and has provided these services for over 38 years. PACS will accept proposals for short courses, consultants, activated carbon conference directors for focused conference subjects, and other needed services. New ideas are welcomed.
PACS, Inc.
409 Meade Drive
Coraopolis, PA 15108
PACSlabs.com